Research
Introduction
Programmed cell death is an essential property of all multicellular organisms. Already in 1973 Schweichel and Merker described three different cell death morphologies, which are now known as apoptosis, necrosis/necroptosis and autophagy.
Apoptosis, which is also called programmed cell death, is a process that allows the specific elimination of damaged or infected cells without induction of an inflammatory response. This cell death program has an important physiological relevance for T-cell selection, embryonic morphogenesis and tissue homeostasis. A deregulation of apoptosis is linked to cancer, AIDS and neurodegenerative and autoimmune diseases. The study of the detailed molecular mechanisms underlying the apoptotic process is an essential step for the development of new therapies to combat these diseases.
Apoptotic dying cells show typical biochemical and morphological changes like cell detachment, shrinkage, nuclear condensation, DNA fragmentation, membrane blebbing and finally the degradation into apoptotic bodies. These apoptotic cell fragments can be engulfed by neighboring and phagocytic cells without the induction of an inflammatory response.
Necrotic cell death is linked to a strong inflammation due to massive cell content release. Typical signs of necrosis are increase of cell volume, swollen organelles, randomized DNA-degradation, high levels of reactive oxygen species and enlarged nuclei. While necrosis was thought for a long time to be an accidental event it became clear in the last years, that it can be induced by specific stimuli and is under tight control of distinct signaling pathways. The programmed necrosis has been named necroptosis. The induction of necroptosis strongly depends on the kinase activity of Receptor Interacting Protein Kinase (RIPK) 1 and 3 that interact and phosphorylate each other in the Necrosome complex.
Apoptosis signaling The central event of apoptosis is the activation of caspases, cysteine-dependent aspartate-proteases, which cleave a number of substrates that followed by the induction of the typical morphological and biochemical changes of the apoptotic cell. Caspases are divided into the initiator and effector ones. Initiator caspases are activated in high-molecular weight complexes and start the pro-apoptotic signal by proteolytic activation of effector caspases. This leads to initiation of a caspase cascade resulting in apoptotic cell death.
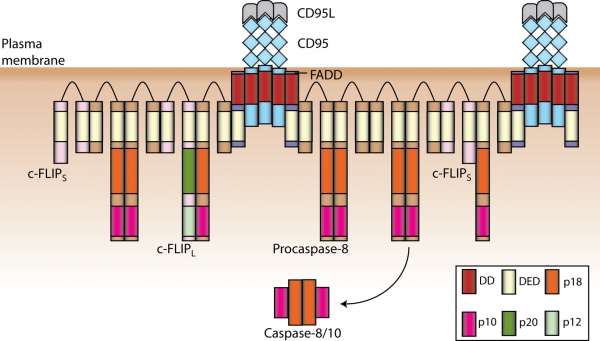
Apoptosis can be induced by growth factor withdrawal, signaling from chemotherapeutic drugs and stimulation of the death receptors (DR). DRs belong to the tumor necrosis factor receptor (TNF-R) super family of proteins and initiate apoptosis upon stimulation with their respective ligands. All DRs are characterized an intracellular approximately 80 amino acid long motif, the so called death domain (DD). DD plays a central role in the transduction of the apoptotic signal. CD95/Fas(APO-1) is the best-characterized DR. Stimulation of CD95 leads to the formation of the DISC (Death-Inducing Signaling Complex). CD95 DISC comprises CD95, adaptor protein FADD, procaspase-8, procaspase-10 and c-FLIP. Interactions between the molecules in the DISC are mediated by homotypic motifs. FADD is recruited via DD interactions with the receptor, procaspase-8/10 and c-FLIP are recruited to the DISC via death effector domain (DED). Recently, we have demonstrated that procaspase-8 activation at the DISC takes place via the DED chains at the DISC. These ’chains of death’ are formed by interactions of DED domains and provide an excellent platform for dimerization and subsequent activation of procaspase-8 (Schleich et al., 2012, Mol Cell), see the figure-.
Our Research
The research programme involves scientific projects on the regulation of Apoptosis, Inflammation and Cancer using Systems Biology. Central point of our investigations is the control of the cell death and switching between apoptotic and non-apoptotic phenotypes in death receptor system. Special attention is given to the assembly and composition of protein complexes as well as to the role of posttranslational modifications in the regulation of death receptor signaling pathways. In addition, we are interested in the spatio-temporal control of the cell death events which is approached by single cell analysis. Our research programme is strongly focused on Systems Biology studies of death receptor networks. This analysis combines mathematical modeling with the biochemical knowledge of the death receptor pathways and allows understanding life/death decisions in the cell on the quantitative level. Deregulation of apoptosis and inflammation is associated to a number of diseases, such as cancer, autoimmune diseases and others. Our research programme is also aimed at unraveling the defects in death receptor-mediated apoptosis and inflammation associated to these diseases. This might lead to discovery of new targets based on death receptor pathways and provide an important basis for pharmaceutical drug development.
Areas of research
- Apoptosis regulation by systems biology
- Regulation of apoptotic and non-apoptotic signaling in death receptor system
- Spatio-temporal control of death receptor signaling
- Control of the NF-kB system by death receptors
- Dynamics and function of death receptor networks - mathematical modeling/systems biology








